-
0 前言
-
H62黄铜作为一种良好的导电性和导热性、高硬度和强度、良好的耐蚀性的金属材料,广泛应用于电子和电气、机器制造业和国防工业等领域 [1-3],然而,它在Cl− 等腐蚀环境中极易腐蚀,据报道,高Cl− 浓度将致使火电厂凝汽器黄铜管腐蚀加剧,进而造成相应的经济损失和引发安全事故[4-6],在H62黄铜表面涂覆耐蚀涂层是提升其耐蚀性能的有效方法。单独的环氧等树脂涂层由于存在大量的微孔呈现出较差的缓蚀性能,因此添加纳米增强相是进一步提升其缓蚀性能的可行路径。
-
石墨烯具有由sp 2 杂化碳原子组成的平面蜂窝状结构,其独特结构使其具有优异的导电性、导热性和润滑特性[7],除此之外,石墨烯由于其具有大的比表面积、高的化学惰性以及优异的阻隔性,在放腐蚀涂层领域受到了表面工程界的青睐[8-9]。然而,石墨烯在环氧、聚氨酯等树脂中由于片层间强烈的相互作用极易发生团聚,在树脂中难以均匀分散,虽然加入分散剂在一定程度上降低了石墨烯片层间的强相互作用,但是在环氧、聚氨酯等树脂中仍难以达到完全均匀分散[10]。此外,虽然目前石墨烯的成本较低,但是石墨烯工程化的相关核心技术仍未突破。基于此,研发分散性好的耐蚀材料具有重要的实际意义。
-
锑碳纳米材料是一种性能优异的锂离子电池负极材料,但对于其耐蚀性的探究鲜有报道。本文采用电化学电解锑的方法在硫酸钠、乙二醇混合电解液中成功制备出水溶性锑碳纳米材料,考察了不同锑碳纳米材料对水性聚氨酯涂层耐蚀性能的影响,探究锑碳/聚氨酯复合涂层的耐蚀机理。
-
1 试验
-
1.1 材料
-
尺寸为5cm × 3cm × 2cm的锑块(纯度99.999%)和1cm × 1cm × 0.1铂电极购于冠泰金属材料有限公司,氯化钠、硫酸钠、乙二醇购于阿拉丁,均为分析纯。水性聚氨酯购于吉田化工。尺寸为3cm × 3cm × 1cm的H62黄铜块购于深圳市质宝金属制品有限公司。
-
H62黄铜的化学成分(质量分数)如表1。
-
1.2 锑碳(Sb-C)纳米复合材料的制备
-
电压设定为10V,锑块和铂电极作为阴极和阳极在50ml的0.5M的Na2SO4 和50ml乙二醇混合电解液中电解剥离12h,将得到的产物通过离心、抽滤等步骤,最终得到水溶性Sb-C纳米材料(如图1)。
-
图1 Sb-C纳米复合材料水溶液
-
Fig.1 Aqueous solution of Sb-C nanocomposite
-
1.3 涂料、涂层制备
-
首先将10g水性聚氨酯添加至50ml烧杯中,分别将0、0.1、0.2、0.3g锑碳纳米材料添加至水性聚氨酯中,添加适量的消泡剂后在1 000r/min的转速下快速搅拌5h,所得物质即为锑碳/聚氨酯复合涂料。将制备的涂料喷涂在H62黄铜基底表面,在60 °C的真空干燥箱中干燥24h即得聚氨酯、锑碳/聚氨酯复合涂层,所有涂层厚度均约为10 μm。
-
1.4 材料的表征
-
采用SU8010扫描电镜(SEM)和TF20透射电镜(TEM)分析锑碳纳米材料微观结构和形貌H62黄铜基底表面涂层腐蚀测试后,测试样品的截面在镶嵌机里用树脂通过高温高压制成树脂圆片,经过抛光后由扫描电镜表征其截面形貌。物相组成由X射线衍射仪(XRD)分析,拉曼光谱由Raman光谱仪测试,激发波长为532nm,扫描范围0~3 500cm−1。
-
1.5 耐蚀性能测试
-
采用上海辰华CHI660E型电化学工作站测试样品的极化曲线与电化学阻抗谱,饱和Ag/AgCl电极作为参比电极,铂电极作为附属电极,H62黄铜块作为工作电极。首先进行30min的开路电位测试,在稳定的开路电位下进行极化测试和电化学阻抗谱测试,测试溶液体系为3.5wt.%NaCl溶液。极化测试条件是扫描速率为0.005V/s,测试电位为 –0.5~–0.05V;电化学阻抗谱的测试条件是振幅为0.7V,频率范围为0.01~105 Hz。
-
2 结果与讨论
-
2.1 Sb-C纳米材料表征分析
-
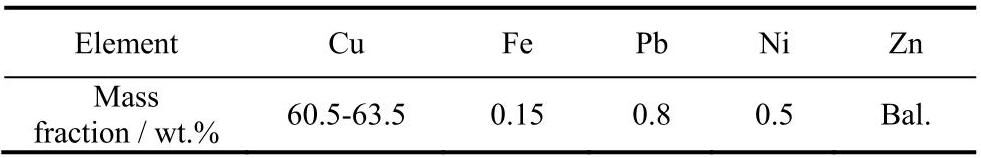
图2a为电化学剥离物质的XRD图谱,衍射峰13.89°、27.7°、32.1°、35.1°、45.9°、54.5°、57.2° 和59.1°分别对应立方相Sb2O3(1 1 1)、(4 0 0)、(3 3 1)、(4 4 0)、(6 2 2)、(4 4 4) 和 (7 1 1) 面,与PDF标准谱图对比可知,剥离物质为立方相Sb2O3 (PDF#05-0534)。图2b为剥离物质的Raman光谱图,其振动峰峰位99、144、188、249和448cm−1 对应于立方相Sb2O3 特征峰[11]。此外,峰位位于1 393和1 598cm−1 的振动峰对应于无定型碳的特征振动峰[12-13]。Raman测试结果显示,电化学剥离过程中乙二醇在电化学作用下为涂层中引入无定型碳。
-
图2 Sb-C纳米材料谱图
-
Fig.2 XRD pattern and Raman spectra of Sb-C nanocomposite
-
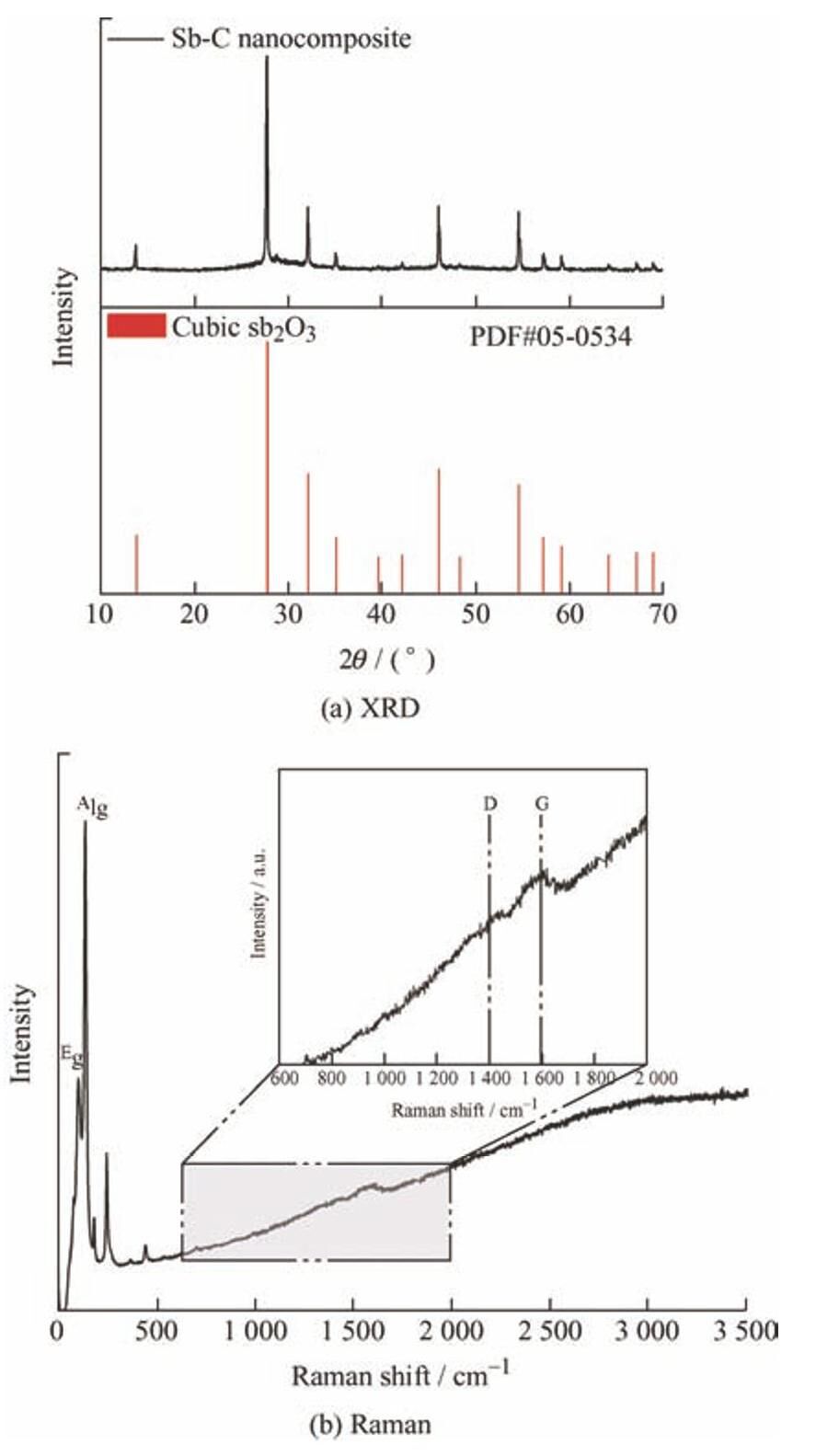
图3a为Sb-C纳米材料的扫描电镜图,Sb-C纳米复合材料微结构呈层状和花瓣状,相应的EDS元素分析谱(图3b~3d)显示,Sb-C纳米复合材料主要成分为碳,还有少部分氧和锑。
-
图3 Sb-C纳米复合材料微观结构及元素谱图
-
Fig.3 Microstructure and element spectra of Sb-C nanocomposite
-
图4a为Sb-C纳米材料的透射电镜图,图4b为图4a中白色正方形标记区域的高分辨透射电镜图,晶格间距为0.322nm的晶体对应于立方相Sb2O3 (2 2 2)晶面(PDF#05-0534),晶体分散于无定型碳网络中。分析结果与Raman测试结果相符。
-
图4 Sb-C纳米复合材料的透射电镜图
-
Fig.4 TEM image of Sb-C nanocomposite
-
2.2 锑碳/聚氨酯复合涂层及其耐蚀性能
-
2.2.1 极化测试
-
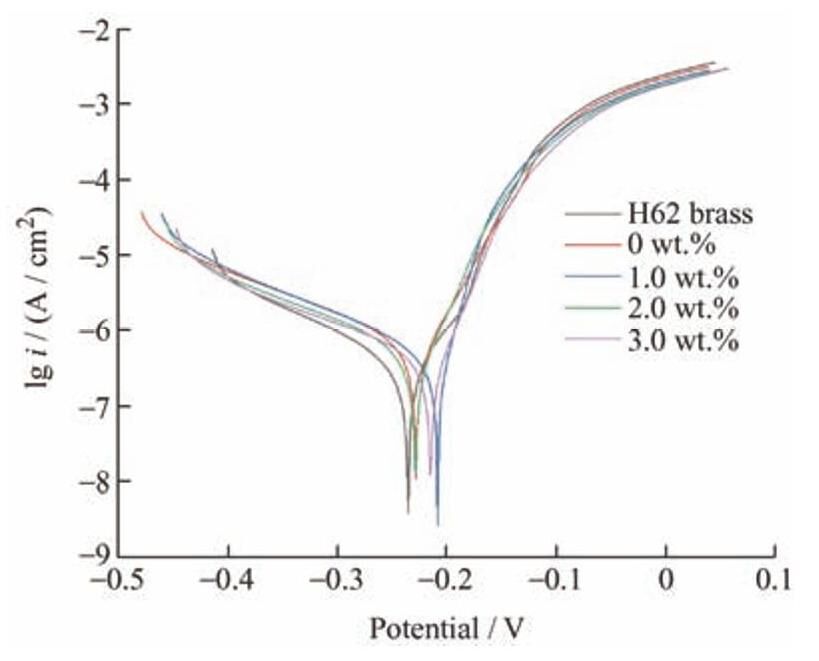
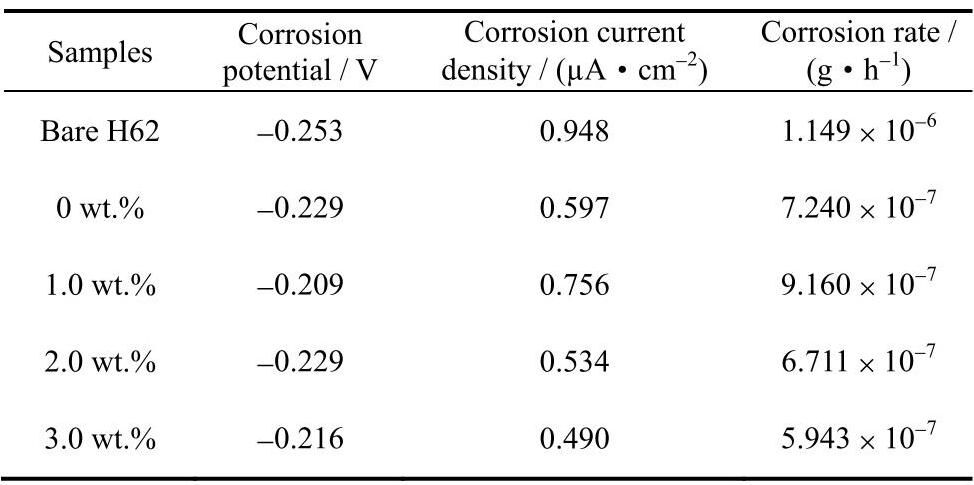
图5 为H62黄铜基底,涂覆聚氨酯和锑碳/聚氨酯复合涂层的H62黄铜的极化曲线图。涂覆了涂层的测试样品腐蚀电位均向右移动,腐蚀电流密度均向下移动,具体极化测试数据如表2所示,当锑碳纳米材料添加量为1.0wt.%时,相比于黄铜基底,腐蚀电位正移44mV,腐蚀电流密度下降约1.3倍,腐蚀速率下降约1个数量级,当锑碳纳米材料添加量为2.0wt.%时,相比于黄铜基底,腐蚀电位正移24mV,腐蚀电流密度下降约2倍,腐蚀速率下降约2个数量级,当锑碳纳米材料添加量为3.0wt.%时,其腐蚀与H62黄铜基底相比正移37mV,腐蚀电流密度下降约2倍,腐蚀速率下降约2个数量级。另外,相比于1.0wt.%,锑碳纳米材料添加量增大至2.0wt.%和3.0wt.%后,除了腐蚀电位负移20和7mV外,腐蚀电流密度和腐蚀速率均发生正移,说明锑碳纳米材料的加入能有效提升复合涂层的耐蚀性能。
-
图5 锑碳/聚氨酯复合涂层极化测试曲线
-
Fig.5 Potentiodynamic polarization curve of Sb-C/polyurethane composite coating
-
2.2.2 阻抗谱测试
-
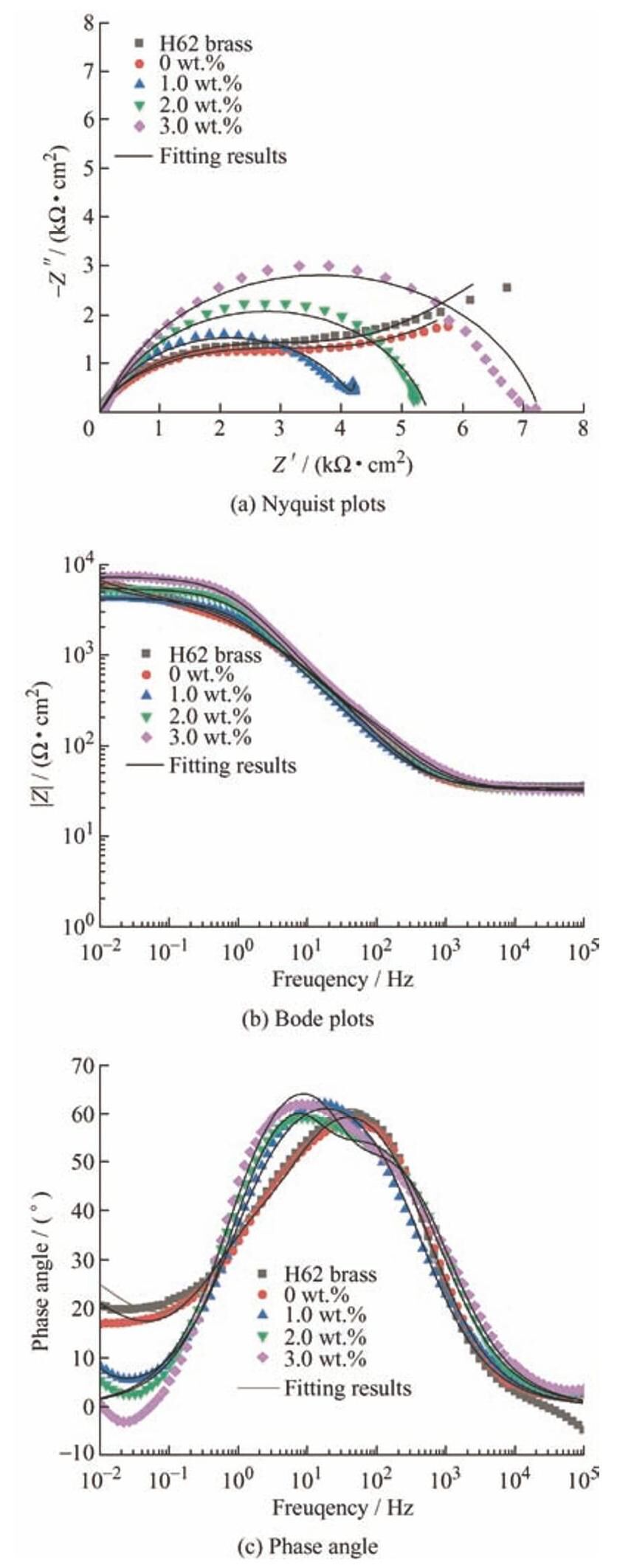
图6 为测试样品的Nyquist和Bode图。由图6a可知,对于H62黄铜基底和聚氨酯涂层,容抗弧出现在高频区,容抗弧代表溶液与工作电极间的电荷转移过程[14],在低频区出现明显的Warburg阻抗,Warburg阻抗表示腐蚀介质向工作电极的线性扩散过程[15-16]。随着复合涂层中锑碳纳米材料含量的增加,阻抗的实部和虚部也逐渐增大。图6b显示,随着锑碳纳米材料含量的增加,阻抗模值逐渐增大。图6c为相位角Bode图,在高频区域主要为容抗响应区,主要产生于腐蚀产物膜聚氨酯涂层、锑碳/聚氨酯复合涂层对腐蚀介质的阻隔效应[16]。
-
图6 测试样品的电化学测试结果
-
Fig.6 Electrochemical test results of the tested samples
-
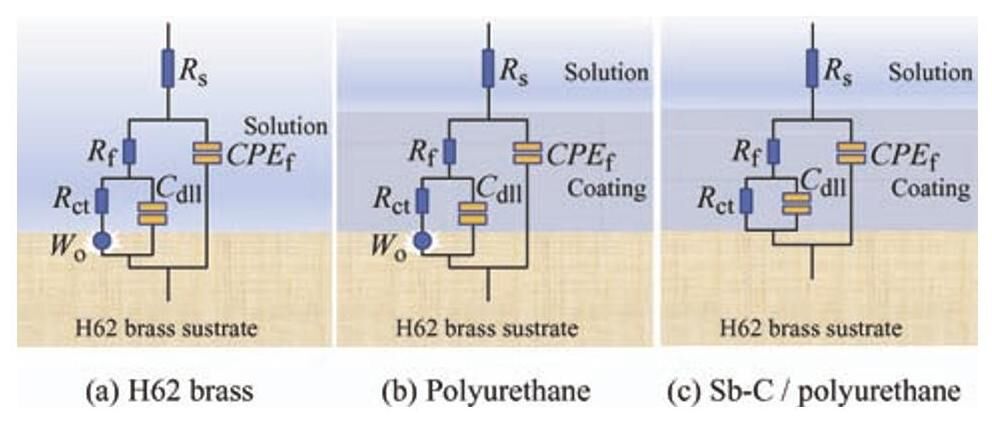
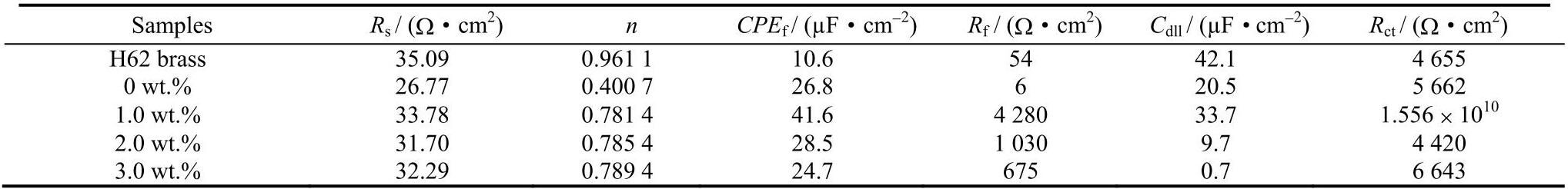
图7a、7b和7c别为H62黄铜基底、聚氨酯涂层和锑碳/聚氨酯复合涂层的拟合电路图,图7a中 R s、R f、R ct 分别为溶液阻抗、腐蚀产物膜阻抗和电荷转移阻抗,CPE f 代表腐蚀产物容抗,C dll 代表电双层电容。拟合电路中相应电阻和电容随锑碳纳米材料含量的变化趋势如表3所示。聚氨酯涂层的涂层阻抗 R f 值最小,说明聚氨酯涂层存在大量微孔,Cl− 可以穿过这些微孔与H62黄铜基底发生电化学反应,对应于图7b中的Warburg阻抗。当添加锑碳纳米材料到聚氨酯中形成复合涂层时,复合涂层阻抗 R f 值增大,说明锑碳纳米材料有效地填补了聚氨酯涂层中微孔,当添加量为1.0wt.%时,R f 值最大(4 280 Ω·cm 2),此时的 R ct 值达到15.56GΩ·cm 2,有效地阻隔了Cl− 向涂层渗透,从而提升了复合涂层的耐蚀性。当进一步增加锑碳纳米材料的含量时,R f 值逐渐降低,R ct 值先降后增。与 R f 值相比,R ct 是决定复合涂层耐蚀性能的关键因素。
-
图7 阻抗谱拟合电路图
-
Fig.7 Equivalent electrical circuits for the impedance plots of (a) H62brass (b) polyurethane (c) Sb-C/polyurethane
-
R s: Solution resistance; n: Coefficient of capacitive reactance; CPE f: Film capacitor; R f: Film resistance; C dll: Electric double layer capacitor; R ct: Charge transfer resistance.
-
2.3 耐蚀机理分析
-
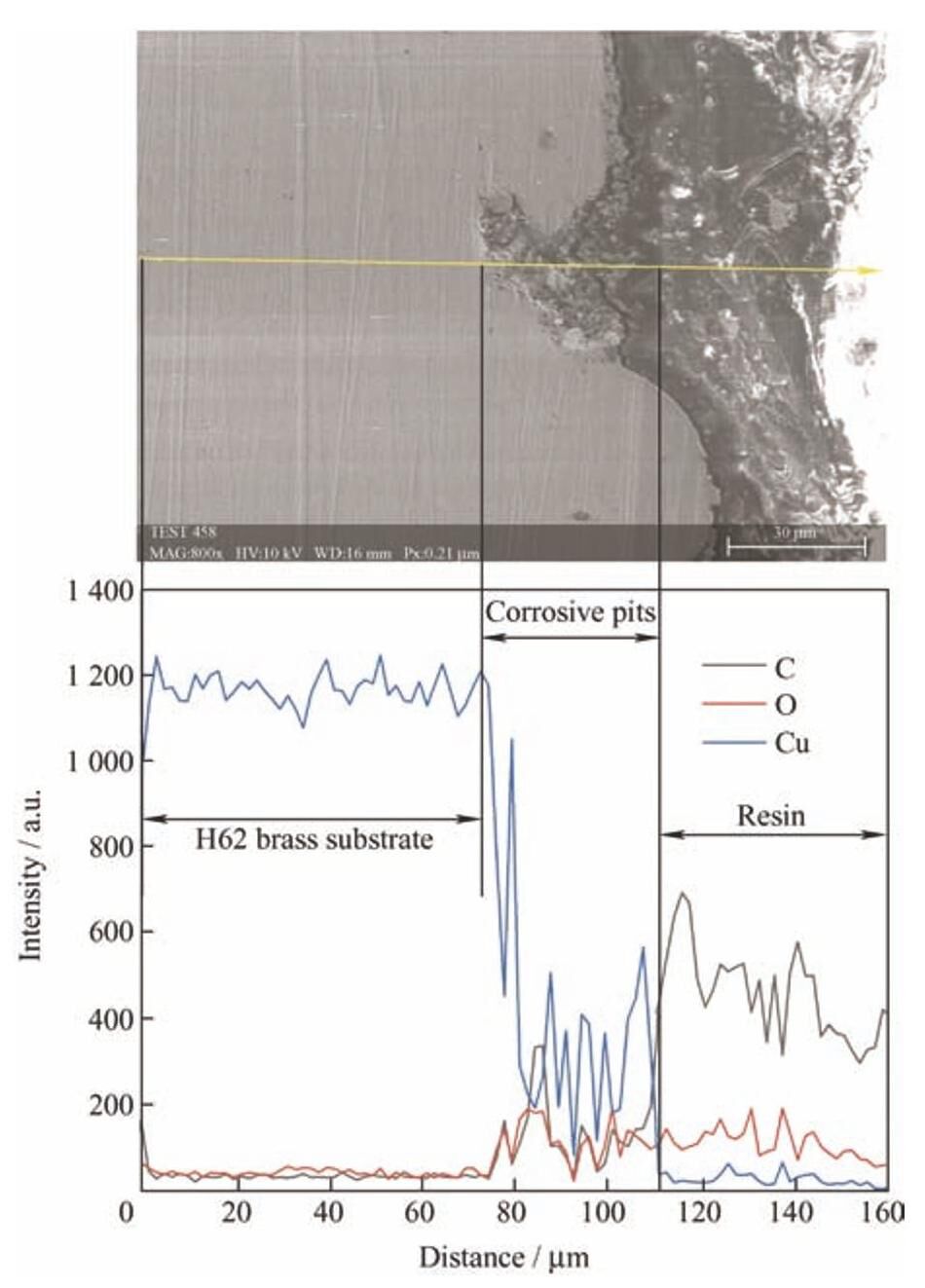
基于以上阻抗谱分析结果,将重点探究锑碳添加量为1.0wt.%的锑碳/聚氨酯复合涂层的耐蚀机理。如图8所示,H62黄铜基底在3.5wt.%NaCl溶液中浸泡72h后,截面出现严重的腐蚀坑,腐蚀坑内的物质由EDS可知主要为铜的氧化物,说明基底与腐蚀介质间发生了电化学反应,结合电化学阻抗谱测试结果,腐蚀产物(铜的氧化物)膜的生成有效地阻隔了腐蚀溶液与基底的接触,阻断了腐蚀溶液与基底间的电荷转移。
-
图8 H62黄铜基底在3.5wt.%NaCl溶液中浸泡72h后截面扫描电镜图及相应的EDS元素分布
-
Fig.8 SEM image and EDS curve of H62brass substrate immersion in 3.5wt.%NaCl solution for 72h
-
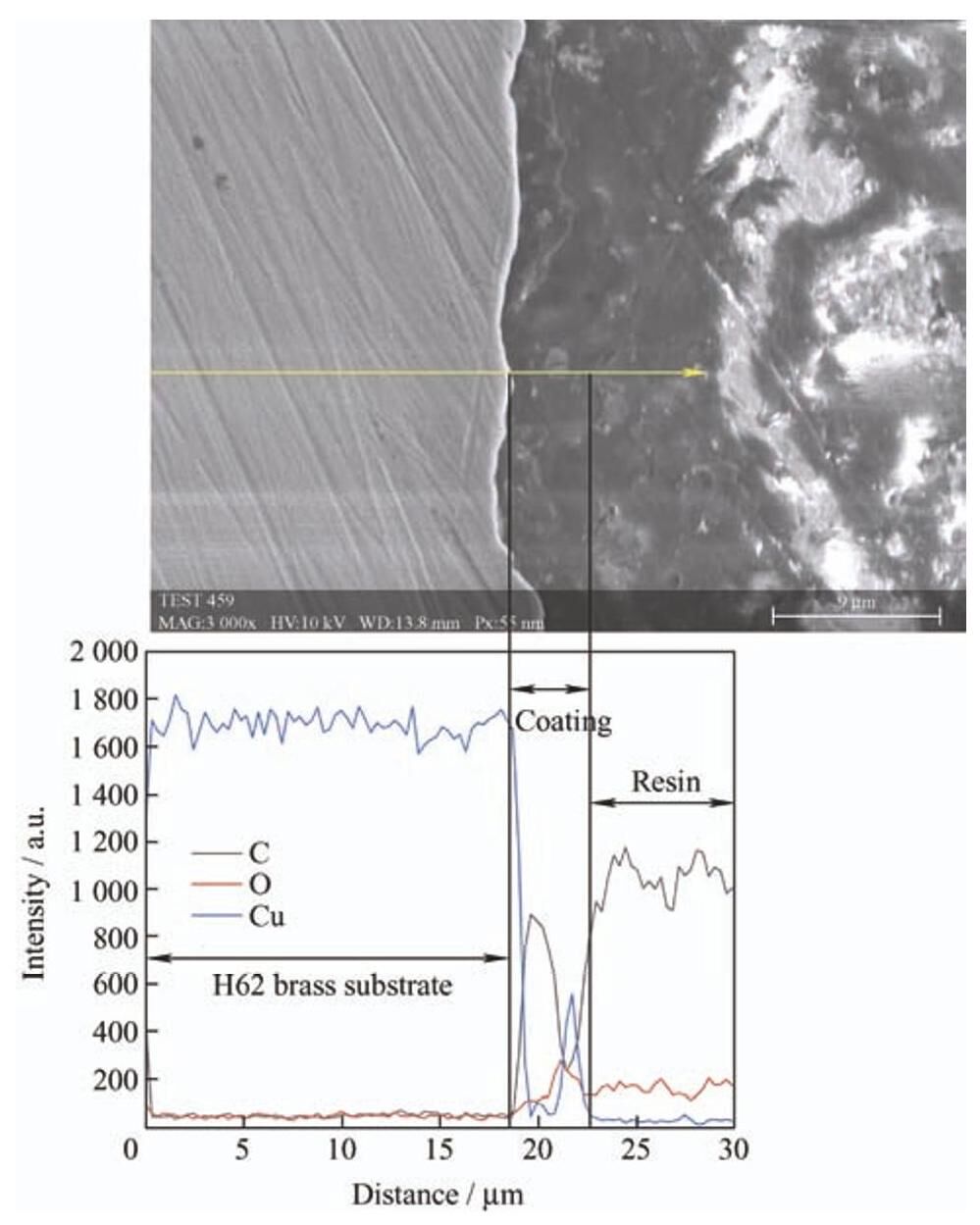
图9 为涂覆聚氨酯涂层后H62黄铜基底在3.5wt.%NaCl溶液中浸泡72h后的截面扫描电镜图和相应的EDS,截面较平整并无明显的腐蚀坑,但由EDS可知,存在铜的氧化物,说明腐蚀介质Cl− 已经穿透涂层与黄铜基底发生了电化学反应。
-
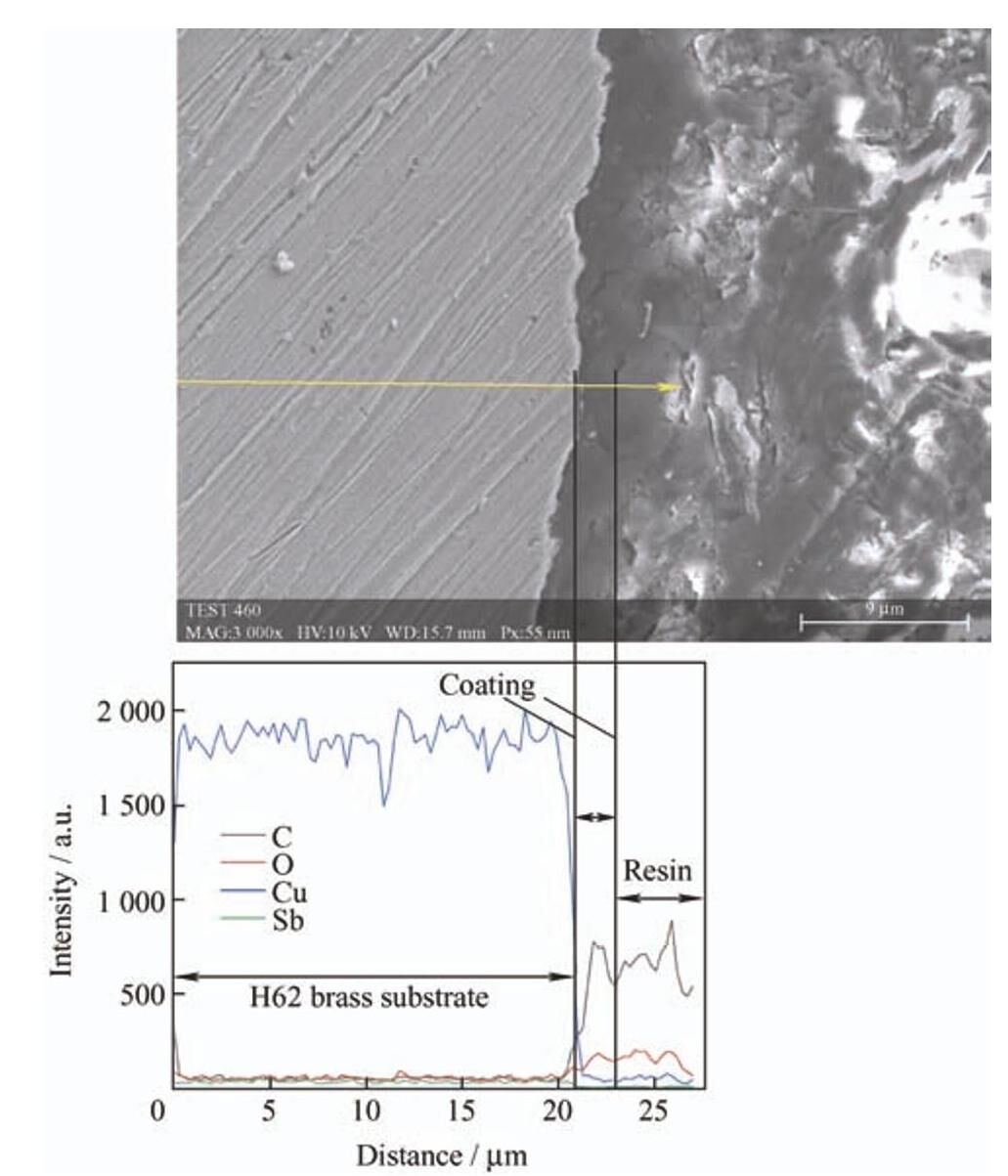
如图10所示,涂覆锑碳添加量为1.0wt.%的锑碳/聚氨酯复合涂层的H62黄铜基底截面扫描电镜图显示:截面处无明显腐蚀,EDS显示界面处无铜的氧化物,结合阻抗谱分析结果,当添加量为1.0wt.%时,R f 值最大(4 280 Ω·cm 2),此时的 R ct 值达到15.56GΩ·cm 2,有效地阻隔了Cl− 向涂层内部渗透。EDS未检测到Sb,这是由于所制备的Sb2O3 为水溶性氧化物,在浸泡过程中溶解在NaCl溶液中作为缓蚀剂阻隔腐蚀介质Cl−,另外所制备锑碳纳米材料中的层状碳很好的填补了聚氨酯涂层中的微孔,提升了复合涂层的耐蚀能力。
-
图9 涂覆聚氨酯涂层的H62黄铜基底在3.5wt.%NaCl溶液中浸泡72h后截面扫描电镜图及相应的EDS元素分布
-
Fig.9 SEM image and EDS curve of the polyurethane coating coated H62brass substrate immersion in 3.5wt.%NaCl solution for 72h
-
图10 质量分数为1.0%的锑碳/聚氨酯复合涂层在3.5wt.%NaCl溶液中浸泡72h后截面扫描电镜图及相应的EDS元素分布
-
Fig.10 SEM image and EDS curve of the Sb-C/polyurethane composite coating with 1.0wt.%Sb-C nanocomposite immersion in 3.5wt.%NaCl solution for 72h
-
3 结论
-
采用电化学剥离方法成功制备水溶性锑碳纳米材料,与聚氨酯混合制备得到锑碳/聚氨酯复合涂料,通过在H62黄铜表面涂装锑碳/聚氨酯复合涂料制备了锑碳/聚氨酯复合涂层,系统探究了其耐蚀性能。本研究成果的主要优势主要有:
-
(1)首次在乙二醇/硫酸钠混合电解液中剥离制备锑碳纳米材,Sb2O3 颗粒均匀分散在层状碳表面。
-
(2)锑碳纳米材料能均匀地分散在水性聚氨酯中,电化学极化测试结果显示,锑碳纳米材料的添加能有效地增大聚氨酯涂层的腐蚀电位和降低腐蚀电流密度。
-
(3)电化学阻抗谱测试结果显示,涂层的耐蚀性能与 R ct 密切相关,锑碳纳米材料添加量为1.0wt.%时,R ct 值最大,具有最强阻断黄铜基底与腐蚀溶液间的电荷转移能力。
-
参考文献
-
[1] LU X,LIU Y W,ZHAO H T,et al.Corrosion behavior of brass H62 in harsh marine atmosphere in Nansha islands,China[J].Journal of Materials Engineering and Performance,2020,29(12):8156-8164.
-
[2] SUN X Y,CHEN Z Y,LI J R,et al.Initial NaCl induced atmosphere corrosion of a dual-phase Cu60-40Zn alloy⎯effect of UV illumination[J].International Journal of Electrochemical Science,2018,13(8):8150-8169.
-
[3] 陈浩,张涛,房文轩,等.500 kV 变电站罐式断路器黄铜连接螺母腐蚀断裂原因分析[J].热加工工艺,2022,51(4):152-154,158.CHEN Hao,ZHANG Tao,FANG Wenxuan,et al.Corrosion failure analysis of brass nut of tank circuit breaker for 500 kV substation[J].Hot Working Technology,2022,51(4):152-154,158.(in Chinese)
-
[4] 齐东梅,成若义,杜小青,等.Cu 及其合金的大气腐蚀研究现状[J].中国腐蚀与防护学报,2014,34(5):389-398.QI Dongmei,CHENG Ruoyi,DU Xiaoqing,et al.Review on atmospheric corrosion of copper and copper alloys[J].Journal of Chinese Society for Corrosion and Protection,2014,34(5):389-398.(in Chinese)
-
[5] 王志武,原素芳.黄铜腐蚀速度与Cl-浓度的关系[J].材料保护,2004,37(10):50-51.WANG Zhiwu,YUAN Sufang.Relation between corrosionrate of brass and concentration of chloride ion[J].Materials Protection,2004,37(10):50-51.(in Chinese)
-
[6] JONG D,WIM H,RIJK De,et al.Toxicity of copper oxide and basic copper carbonate nanoparticles after short-term oral exposure in rats[J].Nanotoxicology,2019,13(1):50-72.
-
[7] 孔尚,胡文敬,李久盛.石墨烯在PAO基础油中的摩擦学性能[J].中国表面工程,2019,32(3):162-169.KONG Shang,HU Wenjing,LI Jiusheng.Tribology property of graphene in PAO base oil[J].China Surface Engineering,2019,32(3):162-169.(in Chinese)
-
[8] 耿浩,李金华,刘宣勇.石墨烯在表面工程领域的研究进展[J].中国表面工程,2015,28(1):4-14.GENG Hao,LI Jinhua,LIU Xuanyong.Research progress on graphene in surface engineering[J].China Surface Engineering,2015,28(1):4-14.(in Chinese)
-
[9] LI HAO,PU J B,ZHANG R H.Long-term corrosion protection of Q235 steel by graphene oxide composite coating[J].Surface Topography:Metrology and Properties,2019,7:045022.
-
[10] CUI M J,DONG J D,ZHOU K H,et al.Corrosion protection of water-borne epoxy coatings incorporated with graphene[J].International Journal of Electrochemical Science,2018,13(12):12010-12023.
-
[11] CODY C A,DICARLO L,DARLINGTON R K.Vibrational and thermal study of antimony oxides[J].Inorganic Chemistry,1979,18(6):1572-1576.
-
[12] 孙薇薇,田修波,李慕勤,等.偏压对自源笼形空心阴极放电制备 Si-DLC 薄膜结构和性能的影响[J].中国表面工程,2019,32(3):69-79.SUN Weiwei,TIAN Xiubo,LI Muqin,et al,Effects of bias voltage on structure and property of Si-DLC films fabricated by self-source cage type hollow cathodedischarge process[J].China Surface Engineering,2019,32(3):69-79.(in Chinese)
-
[13] ZHANG R H,SHEN M X,HE Z Y.The unusual tribological behavior of diamond-like carbon films under high vacuum[J].Surface and Interface Analysis,2020,52:339-347.
-
[14] KHADIRI A,OUSSLIM A,BEKKOUCHE K,et al.Inhibition effects on the corrosion of mild steel in 1 M HCl by 1,1'-(2,2'-(2,2'-oxybis(ethane-2,1-diyl)bis(sulfanediyl)bis(etane-2,1-diyl)diazepan-2-one[J].Portugaliae Electrochimical Acta,2014,32(1):35-50.
-
[15] POSUDIEVSKY O Y,KOZARENKO O A,DYADYUN V S,et al.Effect of host-guest versus core-shell structure on electrochemical characteristics of vanadium ocide/polypyrrole nanocomposites[J].Electrochimica Acta,2011,58:442-448.
-
[16] CASERO E,PARRA-ALFAMBRA A M,PETITFOMINGUEZ M D,et al.Differentiation between graphene oxide and reduced graphene by electrochemical impedance spectroscopy(EIS)[J].Electrochemisty Communications,2012,20:63-66.
-
[17] TARYBA M,LAMAKA S V,SNIHIROVA D,et al.The combination use of scanning vibrating electrode technique and micro-potentiometry to assess the self-repair processes in defects on “smart” coatings applied to galvanized steel[J].Electrochimical Acta,2011,56:4475-4488.
-
摘要
H62 黄铜因其良好的加工性能和较好的传热性能,常用于冷凝器的热交换材料,但是在使用过程中易于发生腐蚀。为了进一步提升 H62 黄铜的耐蚀性能,采用电化学剥离的方法成功制备得到锑碳纳米材料,与聚氨酯混合制备得到锑碳 / 聚氨酯复合涂料,通过在 H62 黄铜表面涂装锑碳 / 聚氨酯复合涂料制备锑碳 / 聚氨酯复合涂层,并利用扫描电子显微镜、电化学工作站、浸泡等表征手段探究涂层的表界面形貌和耐蚀性能。结果表明:与聚氨酯涂层相比,锑碳 / 聚氨酯复合涂层在腐蚀测试过程中具有更正的腐蚀电位(−0.209 V)、高阻抗模值和阻抗值(~7.0 kΩ·cm2 );铜基底表面无明显腐蚀,呈现优异的防护性能。这主要归因于锑碳纳米材料的加入有效地降低了涂层的孔隙率和延缓了腐蚀介质的渗入,从而提高了 Cu 基底的抗腐蚀性能。首次使用有机+无机(乙二醇+硫酸钠)混合电解液成功电解出水溶性锑碳纳米材料,能很好地分散于水性聚氨酯中,在聚氨酯涂层中起到良好的密封和阻隔作用,可为进一步提升 H62 黄铜的耐蚀性提供参考。
Abstract
H62 brass is often used as the heat exchange material for condenser due to its good process performance and thermal conductivity, however, which is easily corroded during application. In order to further improve the anticorrosion performance of H62 brass, Sb-C nanomaterial is fabricated by using electrochemical exfoliation method, Sb-C/polyurethane composite paint is fabricated by mixture of Sb-C composite and polyurethane, the Sb-C/ polyurethane composite coating is fabricated on H62 brass surface by using Sb-C/polyurethane composite paint, and the surface and interface morphologies and anticorrosion performance of the coating are performed by using scanning electron microscope (SEM), electrochemical station and immersion method. The results exhibit that compared to the polyurethane coating, the Sb-C/ polyurethane composite coating shows more positive potential (−0.209 V), high impedance modulus and coating resistance (~7.0 kΩ·cm2 ). Furthermore, no obvious corrosion is observed at the Cu substrate, revealing the outstanding corrosion resistance. It is attributed to that Sb-C nanomaterial can effectively reduce the porosity and prohibit the penetration of the corrosive medium, which is conducive to enhancing the corrosion resistance. The organic-inorganic mixed electrolytes (ethylene glycol and sodium sulphate) is first innovatively used to fabricate water-solubility Sb-C nanomaterials, which can be evenly dispersed in water-solubility polyurethane and play an important role in sealing and barrier for polyurethane coating, giving a necessary reference for further investigating the anti-corrosion of H62 brass.
Keywords
spray ; composite coating ; exfoliation ; microstructure ; anticorrosion property